
Empowering YOUR Healthcare Providers
Crafted RVF® Technology – Engineered with Passion
About the patented RVF® Technology
What is COVID-19?
Invented to detect total antibodies to SARS-CoV-2 and support healthcare providers
SARS-CoV-2 belongs to the Betacoronavirus genus that also includes SARS-CoV and MERS-CoV coronaviruses that caused 2003 and 2012 outbreaks, respectively. How SARS-CoV-2 was transmitted to humans is still unknown, however, some studies suggest that the virus came from bats and was possibly passed to humans through an intermediary host.
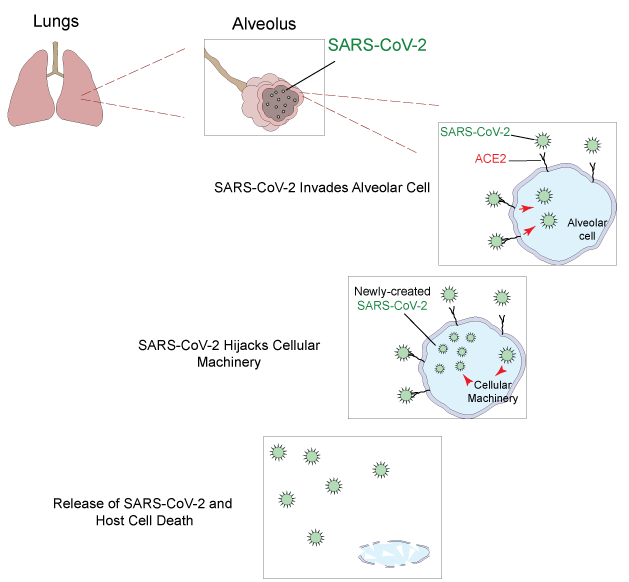
The effect of this novel coronavirus on the human body is not well-understood, but SARS-CoV-2 is known to target the angiotensin-converting enzyme 2 (ACE2) on the surface of the cells that subsequently triggers the import of the viral particles. Upon successful invasion, the virus hijacks the cell maintenance machinery and produces more copies of itself. When the newly created SARS-CoV-2 particles are released, they are free to infect neighboring cells throughout the body. ACE2 presented on the surface of lung alveolar cells is the primary target, however, the effect of SARS-CoV-2 on other organs, such as intestine, heart, brain, and kidneys, is still under investigation.
REVEALCOVID-19™ Total Antibody Test

REVEALCOVID-19™ Total Antibody Test
IS:
- Inexpensive, portable device
- Fast (~3 min) and easy test
- Specialized equipment, additional reagents and skillful workers
- Sophisticated sample preparation
- Widespread testing (including asymptomatic patients)
- Follow-up screening
- Vaccine development
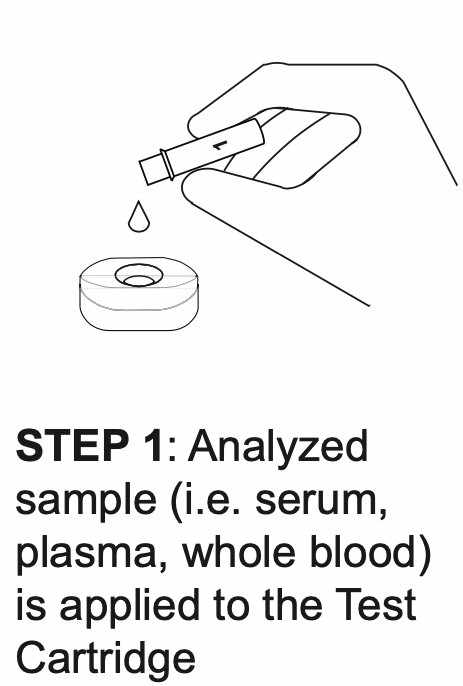
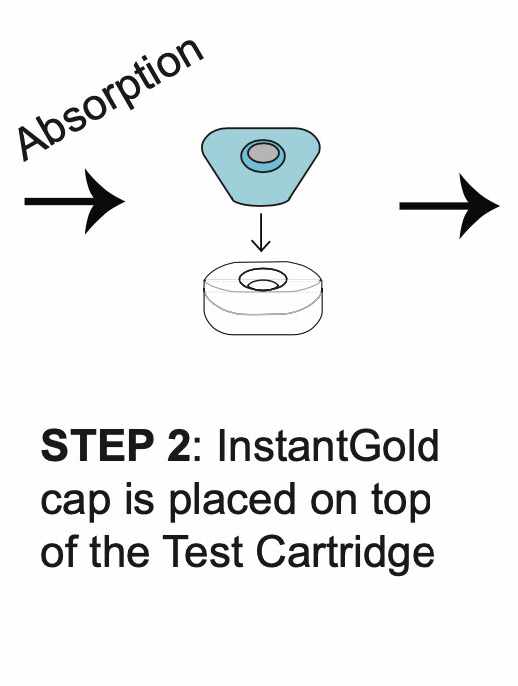
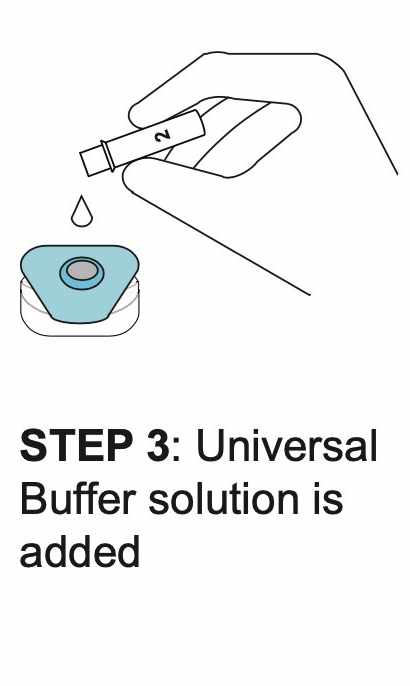
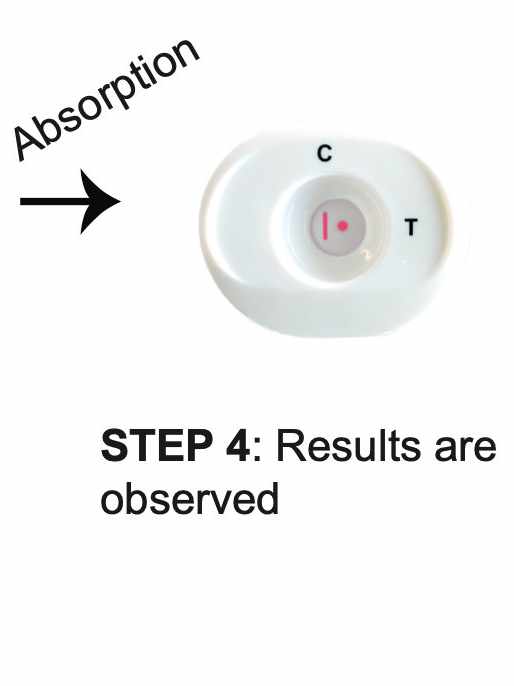
How REVEALCOVID-19™ Total Antibody Test Works?
Packaging Inserts
Disclaimer
- This test has not been FDA cleared or approved.
- This product has not been authorized by FDA under an EUA for use by authorized laboratories.
- This product is not for self-testing.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
- Follow-up testing with molecular diagnostics should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
- Test is not intended for the screening of donated blood
Useful Links – for up-to-date COVID-19 information
FAQ
Contact us now
MedMira is committed to exceeding your needs. Have a question or comment?
The following options can help direct your inquiries to the right MedMira team
us@revealcovid19.com
ce@revealcovid19.com
canada@revealcovid19.com